SYLLABUS
9. Preconceptional and pregnancy care of women with autoimmune diseases
(Advanced level)
Slide 2.
Aim of the study material:
- To learn about the immunology of pregnancy
- To characterise special groups of pregnant women with autoimmune diseases
- To be able to provide a risk assessment during pregnancy for women with autoimmune diseases
- To be able to engage in adequate pre-pregnancy counselling
- To identify specific risks and preventive methods
Slide 3.
Autoimmune diseases occurs far more often in women then in men. The interplay of genetic, environmental and hormonal factors all influence the development of the immune system. Differences between male and female are clear in their susceptibility to autoimmune diseases but also to infectious diseases and cancer types.
Women differ from men in several clinical characteristics of their immune responses which suggest that they have stronger innate and adaptive immune responses.
Some examples for that are:
- Women have 40% less viral load by an acute HIV infection
- Antibody responses to seasonal influenza vaccines are stronger in women
- non-reproductive cancers , such as bladder, bowel, kidney, leukaemia, malignant melanoma, develop more often in male patients
- specific infectious diseases such as Hepatitis B and tuberculosis affect male patients more often
Slide 4.
Women suffer from autoimmune disease more often then men. On this slide we can see the incidence of some autoimmune diseases, such as SLE, RA, autoimmune thyroid diseases, inflammatory bowel diseases, vasculites, autoimmune uveitis by sex. Women seem to be more prone to the development of autoimmune diseases than men.
Slide 5.
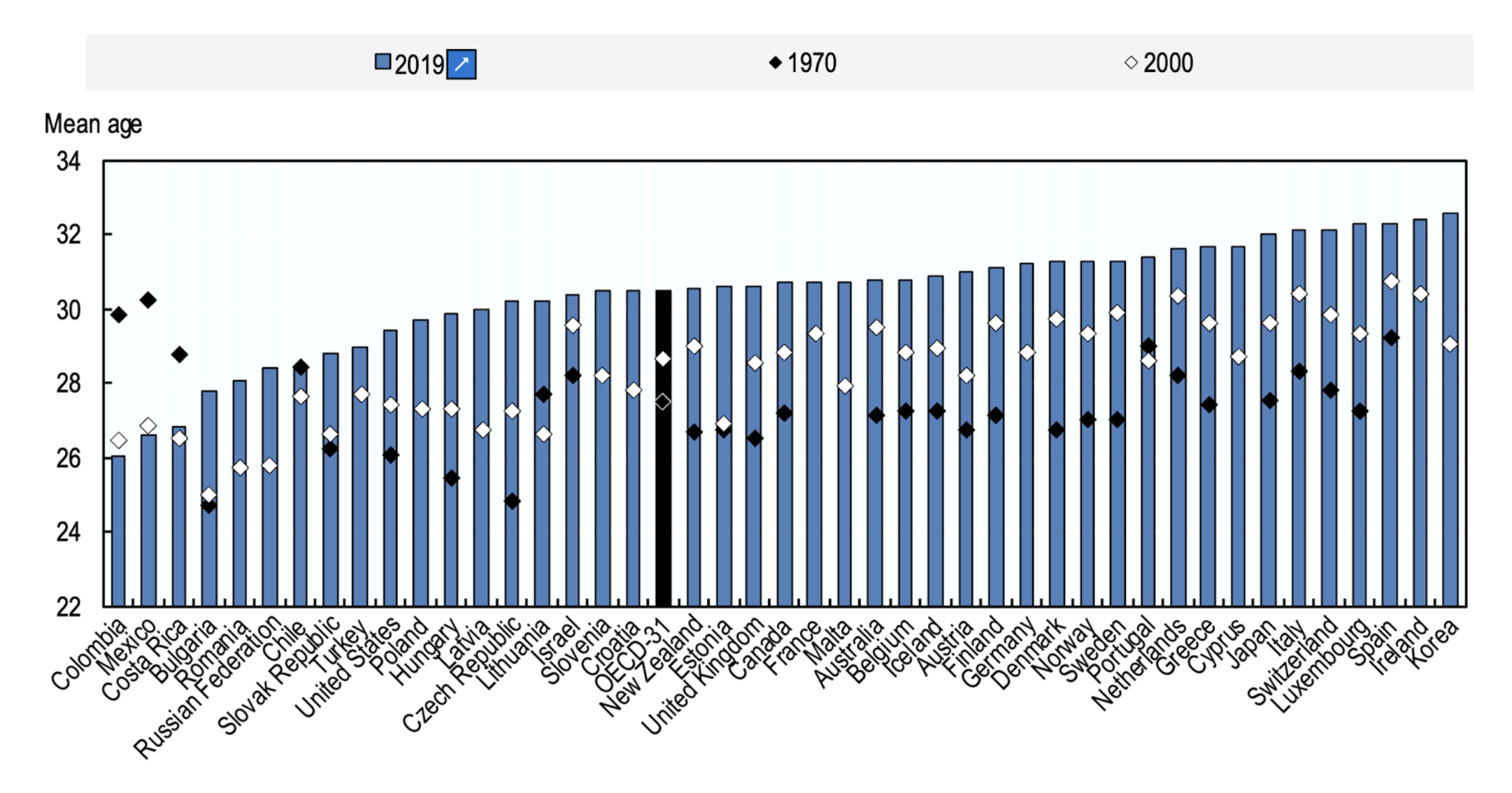
Childbearing age increases gradually every year. (diagram OECD FAMILY DATABASE, oe.cd/fdb)
This trend roots from societal attitudes and expectation, which puts women’s reproductive potential at risk. Most OECD countries the average age of women at childbirth increased by 2 to 5 years between 1970 and 2019, with the largest increase (5.4 years) in the Czech Republic. With the exception of three Latin American countries (Colombia, Costa Rica and Mexico), where the mean age of women at childbirth has decreased by over two years since 1970.
A woman’s peak reproductive years are between the late teens and late 20s. By age 30, fertility (the ability to get pregnant) starts to decline. This decline becomes more rapid once they reach 35 years of age. By 45, fertility has declined so much that getting pregnant naturally is unlikely for most women. Education about the fertility aspects seems important for future generation to fullfill their reproductive potential.
Slide 6
You can see that the number of births over 35 years of age increase steadily between 2004 and 2016, which means that an increasing proportion of women will have an obstetric risk due to their increased age and comorbidities, as well as due to the increasing use of artificial reproductive techniques. This does not mean that fertility itself wasn’t declining with age!
Slide 7
Generally, advanced age comes with common co-morbidities like high blood pressure, diabetes mellitus, autoimmune diseases etc., which might complicate the course of the pregnancy and cause maternal and fetal morbidities. The most common period for the diagnosis of autoimmune diseases lies between th 3rd and 4th decade of life which intersects with family planning and pregnancy.
Women with rheumatic diseases have fewer children than healthy women. Several factors are responsible for this: personal decisions, infrequent sexual intercourse, reduced fertility, fear of taking medication or an active underlying disease.
But depending on the disease, the conditions differ significantly: female patients with inflammatory joint diseases such as rheumatoid arthritis (RA), psoriatic arthritis (PsoA), joint involvement with inflammatory bowel disease (CED), or axial axial spondyloarthropathy (SpA) have, in principle, an average fertility average fertility, but often need a longer time until conception. 25 per cent of patients with RA take longer than 12 months to get pregnant. By comparison, in the healthy control group
rate of subfertility is 15.6 per cent. In women with systemic lupus erythematosus (SLE), fertility is not impaired.
Slide 8
Adaptation of the immune system to pregnancy, different coping in different species:
The different species have developed different strategies of childbearing. Birds, reptiles and fish- for example - take care of their offspring outside of their bodies. This strategy puts the offspring in a greater danger to be a pray for predators, changing temperature and mechanic impacts, but this way the developing offspring does not interfere with the immune system of the mother. In the contrary mammals, - for example humans-, bear their offspring in their body, which provides safety, constant temperature, energy and oxygen supply, however the childbearing animal has to adapt for the period of the pregnancy to tolerate the alloimmune fetus.
Slide 11
What kind of changes happen in the immune system in pregnancy? How do these changes influence the activity of the autoimmune disease?
According to an older theory about the immunological changes during pregnancy, there is a shift between Th1 and Th2 type immune response and Th2 dominance characterises pregnancy. This immunological shift helps to prefer anti-inflammatory cytokine function and through that the maintenance of pregnancy.
Women with autoimmune disease might experience an exacerbation of the underlying disease. Observations suggested that connective tissue disorders (for example SLE or Sjögren´s syndrome) are more likely to flare up during pregnancies, while other autoimmune diseases such as rheumatoid arthritis, juvenile idiopathic arthritis, multiple sclerosis psoriasis arthritis are less likely to gain activity during pregnancy. The reason might lie in the immunological changes causative to the autoimmune disease. Systemic lupus erythematosus can be characterised with an active Th2 type immune cell activation. If the immunological changes during pregnancy also activate these cells, the autoimmune disease might become more active. This theory however does not hold entirely.
Slide 12
New immunological studies showed, that when considering the immunological changes in pregnancy we have to differentiate according to the three phases of the pregnancy. A pro-inflammatory microenvironment is crucial for normal implantation and parturition, whereas a tolerogenic environment is induced during the course of pregnancy to enable normal placentation and fetal growth. The local immunological changes in the placenta and decidua will ensure the success of a pregnancy. The cytokine profile at the decidua is the result of the interplay of fetal cells and hormones, and that the TH1–TH2 paradigm should be replaced by a more complex paradigm involving TH1, TH2, TH17 and Treg cells.
The first phase describes the period from implantation to early placentation. At this point a mainly innate immune cells such as NK cells, dendritic cells, macrophages, neutrophils and type 3 innate lymphoid cells (ILC3s) are responsible for the proinflammatory immune milleu consisting of IFNγ, IL-1, TNF, IL-6, IL-17 and the IL-6 family cytokine leukaemia inhibitory factor (LIF). Pro-inflammatory M1 macrophages secreting IL-23 or IL-12 as well as LIF; decidual NK cells are poorly cytolytic but produce cytokines and chemokines, such as IFNγ and vascular endothelial growth factor, that promote spiral artery remodelling. These factors are essential for successful implantation.
To avoid rejection at the feto-maternal interface in the second phase of the pregnancy a tolerogenic state develops rapidly. Oestrogen, cells and regulatory proteins act on decidual stromal cells and tolerogenic dendritic cells, expand FOXP3+ Treg cells, change the function of the rapidly increasing number of natural killer (NK) cells and downregulate effector T (Teff) cells. Anti-inflammatory cytokine production increases. As a result, the semi allogenic fetus will be tolerated.
In the third stage of the pregnancy- at parturition- again a pro-inflammatory microenvironment is crucial. Innate immune cells, such as neutrophils and macrophages infiltrate the decidua and the chorioamniotic membranes during term labour and secrete matrix metalloproteinases, IL-1, IL-6, TNF and nitric oxide.
Slide 12
All of the above mentioned changes will effect the activity of the autoimmune disease during pregnancy. Well controlled autoimmune diseases have less risk of activity during pregnancy, while active disease periconceptionally poses a risk for flare. This characteristics lies in the nature of the immunological shifts during pregnancy.
Slide 13
This seminar will explain the interplay of pregnancy and autoimmune diseases on the example of inflammatory rheumatic diseases (IRD), which is a large group of different autoimmune diseases. These diseases, tend to be systemic and thus effect the immune response of the patients in its entirety.
By understanding the effect of IRD on pregnancy and vice versa, we can extrapolate the influence of other systemic autoimmune diseases on the course of the pregnancy. However, each individual autoimmune condition might have specific aspects on pregnancy. Unfortunately, this the scope of the seminar does not allow to discuss each systemic autoimmune disease separately.
Autoimmune conditions often affect women of childbearing age and often delay family planning for those affected. The effect of pregnancy on the activity of the autoimmune condition but also the possible influence of the autoimmune condition on the ongoing pregnancy raise concerns and uncertainties in patients. In recent decades, as multiple treatment options have been established that can control disease activity, awareness regarding childbearing in autoimmune diseases has grown. Evidence proves that reaching remission or low disease activity before conception is of utmost importance to minimize risk of flare during pregnancy as well as to minimize the risk of pregnancy complications.
We will focus on systemic lupus erythematosus, and rheumatic arthritis as important examples of IRD in pregnancies. Organ specific autoimmune diseases like M.Basedow, autoimmune thyreoiditis, autoimmune hepatitis will not be discussed here.
Not only the pregnancy will have an effect on the autoimmune disease, but the autoimmune disease also poses a risk on the course of the pregnancy.
Maternal inflammatory phenotype at conception will affect the crucial immunological processes at implantation, which in turn can have a negative effect on placentation, on the development of the spiral arteries and might lead to early miscarriage, preterm rupture of membranes, placental insufficiency, preeclampsia or preterm birth.
Slide 14
In summary we can see complications relating to the underlying rheumatic disease during pregnancy, such as flare, cardial, pulmonal, haematological, renal problems. Some patients experience obstetric or thrombotic or both complications related to antiphospholipid antibodies, which commonly are associated with autoimmune diseases.
On the other hand the prevalence of pregnancy complications, such as preterm birth, intrauterine growth restriction, small for gestational age newborn, preeclampsia are increased in patients with rheumatic diseases.
Antiphospholipid antibodies, when fulfilling clinical an laboratory criteria for antiphospholipid syndrome pose a substantial risk to develop preeclampsia, stillbirth, miscarriage and placental insufficiency. Existing obstetric APLAS should be taken as a serious risk factor in the ongoing pregnancy.
Slide 15
This slide shows on the example of SLE, how the activity of the underlying rheumatic disease affect pregnancy complications. We can see that highly active autoimmune condition elevates the risk of the pregnancy complications. For that reason, preconceptional pregnancy planning is quintessential for risk reduction in the pregnancy.
Slide 16
The particular strength of professional care for pregnant women with inflammatory rheumatic diseases lies in the joint interdisciplinary consultation of a rheumatologist and a gynaecologist. Prepregnancy counselling is important to assess modifiable risk factors and optimize disease activity and therapy before conception.
The following points should be considered in order to optimise the course of pregnancy:
Risk modification by adequate weight control, healthy and balanced diet and abstinence from nicotine, review and change of medications, and risk assessment based on disease activity and antibody profile and previous obstetric history should be done.
Effective contraceptive methods should be suggested until no teratogenic medicaments are given and until the underlying autoimmune condition reaches a steady state in remission for ca 6 months.
The prepregnency care might involve the adaptation of the immunosuppressive medications, might involve increased need of folic acid. Patients after methotrexate therapy or under ongoing sulphasalazine therapy need a significantly higher folic acid substitution (5 mg daily). The substitution should start four to twelve weeks before stopping contraception. In addition, folic acid intake should be continued throughout the entire pregnancy in the case of supphasalazine intake during pregnancy and in the first 13 gestational weeks in the case of methotrexate intake before the pregnancy.
In addition, due to the increased risk of osteoporosis, it is useful to check the serum vitamin D level.
Vaccination status, including antibody titres, should be consistently checked. In the case of inadequate protection against varicella, rubella, measles, mumps, the patient should be vaccinated with a live vaccine before receiving immunosuppressants.
Contraception may only be discontinued one month after vaccination. The flu vaccination and pertussis (inactivated vaccine) is always recommended during pregnancy and should be administered in the second trimester if possible.
Slide 17
Which antibodies are highly relevant in the pregnancy?
For a careful obstetric risk assessment apart form the antibodies, that indicate a possible disease activation antiphospholipid antibody and SSA and SSB Antibodies are relevant for the pregnancy.
Antiphospholipid antibody syndrome (APLAS) can occur as a primary disease or secondary to rheumatic diseases, in particular to SLE. Potential pregnancy complications include recurrent pregnancy loss, an increased risk of hypertension in pregnancy, pre-eclampsia, eclampsia, HELLP syndrome, fetal growth restriction, stillbirth, and thromboembolic events such as venous and arterial venous and arterial thrombosis incl. amaurosis fugax and insults.
Slide 18
For successful pre-conceptional counselling, it is crucial to recognise this syndrome in time. Questions about a history of thrombosis or previous pregnancy complications such as preterm birth, stillbirth, intrauterine growth restriction, recurrent early abortions or late abortion are important. Therapeutically, APLAS can and should be treated in pregnancy with platelet aggregation inhibitors such as ThromboASS® and/ or low-molecular-weight heparins.
Slide 19
SSA/SSB antibodies pose an increased risk of neonatal lupus.
Neonatal lupus (NLE) is and acquired disease, caused by the transplacental transfer of SSA and SSB autoantibodies. There are two forms of NLE: 1. Cutaneous NLE, characterised by a temporary (transient) rash, usually developing during the first few weeks of life and disappearing within 6 months after birth. 2. Cardiac NLE: characterised by fetal and neonatal congenital atrio-ventricular (AV) block. The local inflammatory process caused by SSA and SSB antibodies in the developing fetal heart result in fibrosis of the electrical conduit system and can cause a partial or total dissociation of atrial and ventricle system. The severity of such conduction abnormalities may vary among affected infants meaning there can be first-, second-, or third-degree blocks, the latter most serious. Children with total AV block usually receive a pacemaker. This means that long-term survival is between 87 and 95 percent. Morbidity and mortality is highly influenced by the gestational age at birth.
Slide 20
Pregnant women with SSA/SSB antibodies in the context of Sjögren's syndrome or SLE have a risk (ca.2%) of fetal AV block. In the subsequent pregnancy, the risk of recurrence is much higher with an estimated 20%.
So far no controlled, prospective study on the therapy of congenital AV block is available. This makes prophylaxis all the more important: a retrospective study of 201 pregnant women with SLE showed that pregnant women with SLE showed that hydroxychloroquine reduced the risk of congenital AV block by 50 per cent. In addition, weekly bradycardia screening and fetal echocardiography between the 16th and 28th trimesters are recommended. After birth an ECG of the newborn child should be performed.
Slide 21
Connective tissue disorders, such as SLE and Sjögren syndrom or mixed connective tissue diseases might come with more obstetric complications. For that reason, prepregnancy counselling is of utmost importance.
In cases of active severe disease with SLE, the preterm birth rate is significantly increased at 58 percent (vs. 8 percent in the control group) as well as other pregnancy complications.
Slide 22
Pregnancy is contraindicated, in cases of pulmonary hypertension, active and current neurological and renal involvement, as the mortality of pregnant women is 20-fold higher in this case.
In mild forms of the disease, there is no contraindication for pregnancy, however, clinical remission should be targeted for six months before conception.
Slide 23
As discussed before, optimizing the activity of the underlying autoimmune disease before conception is key to an uneventful course of pregnancy. For that reason, therapeutic intervention and immunosuppression is often required before and throughout pregnancy.
In the last 20 years there was an enormous improvement in the therapy of inflammatory rheumatic diseases. Thanks to the wide availability of biological DMARDS (disease-modifying antirheumatic drugs) many patients can have a symptom free life and feel the need to fulfil their family planning.
This table represents in red the medications, which are contraindicated in pregnancy, green the medications, which can be administered and yellow: the medications, where only insufficient data exist in pregnancy.
Slide 24
Background risiko for early miscarriage and congenital malformation
Even in healthy parents, without any medication, 15 -20% of pregnancies result in early pregnancy loss and 3-5% of these parents give birth to children with congenital malformations. These are statistical facts that have to be communicated to the advice seeking couple as we start or progress with immunomodulation during pregnancy.
There are only a few immunomodulatory medications, which are contraindicated during pregnancy. This means, that they have to be ideally preconceptionally discontinued. Some of these medications persist in the blood stream years after discontinuation. Some of them have substantially shorter half-life.
Professional counselling is needed in the situation when unintentional pregnancy occurs under these therapies. It is always an individual decision to terminate a pregnancy, which has to be based on an interdisciplinary (obstetric, rheumatology, embryology) detailed counselling. Exact time of discontinuation, absolute and relative risks of early miscarriage and congenital malformations need to be stated.
As seen in this table, the relative risks of congenital malformations and miscarriages compared to the basis risk without any medication vary widely among these immunosuppressives.
Slide 25
Medications that are considered safe periconceptually during pregnancy and lactation. This
group include salazopyrin, antimalarials such as Resochin® or Quensyl® and azathrioprine (Imurek®). Salazopyrin® or other 5-ASA preparations are often used for inflammatory
joint disease or inflammatory bowel disease.
A relatively recent finding is that antimalarials not only have a favourable effect on the course of the autoimmune disease but they effectively reduce the risk of NLE.
Disease activity in rheumatoid arthritis, psoriatic arthritis, axial spondyloarthropathy, or inflammatory bowel disease often require intensified therapy with TNF alpha blockers.
Slide 26
Before the registry data became available, it was common to generally advise women against taking biologics during conception, pregnancy or breastfeeding, or lactation.
Common questions from the women planning pregnancy with TNF alpha blockers concern infections of the newborn and possible malformation. Studies does not shoe increased risk for infections or increased incidence of congenital malformations.
Now we know, that TNF alpha blockers, such as certolizumab and etanercept can be used throughout pregnancy. Other TNF alpha blockers have less data in the pregnancy, and can be administered, when benefit at a specific patient outweigh the risk of the medication.
Slide 27
Fetal immunity is acquired during pregnancy by transfer of IgG antibodies from the maternal to the fetal circulation. Of the five major classes of antibodies, only IgG is transferred across the placenta. Analysis of cord sera has shown that all IgG subclasses are transmitted to the fetus but a preferential transport of IgG1 was found. Therapeutic monoclonal antibodies (mAbs) are most commonly of the IgG1 subclass, which is transported most efficiently to the fetus.
Slide 28
In all animal species used for testing developmental toxicity, fetal exposure to IgG is very low during organogenesis, but this increases during the latter half of gestation such that the neonate is born with an IgG1 concentration similar to the mother. Data shows that maternal IgG concentrations increase in fetal blood from early in the second trimester through term, with most antibodies being acquired during the third trimesterr . Only a residual amount of IgG1 antibodies cross the placental barrier during the first trimester. Therefore, they do not have a teratogenic effect.
Slide 29
After birth acquired IgG antibodies slowly degrade from fetal circulation in the first 6-12 months after birth.
Slide 30
Due to the persisting immunomodulation after birth, caution should therefore be exercised with live virus vaccination. This means that live vaccines can be first administered 12 months after birth. This might affect BCG, rotavirus and MMR vaccinations.
Slide 31
In summary, we have to be aware, that:
Family planning should be addressed in every patient with autoimmune disease of reproductive age.
Treatment of patients before/during pregnancy and lactation should aim to prevent disease activity in the mother and not harm the foetus.
The risk of drug therapy to the child should be weighed against the risk of untreated maternal disease to the patient and child.
The decision on drug therapy should be based on an agreement between rheumatologist, gynaecologist and patient in an interdisciplinary and participative manner.